
Steady-State Brusselator with Diffusion
The system is the same as the one in WOBexa1.
Scaling the independent variable according to t:=x/L leads to the system of four ODEs of the first order
with boundary conditions
The ordinary differential equations describe the spatial
dependence of the two chemicals X and Y along a reactor with
length L,
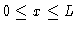 .
This Brusselator model
equation has a great number of solutions ; some are
represented in the branching diagram of Figure 1, which depicts
y2(0)=X'(0) versus
.
This Brusselator model
equation has a great number of solutions ; some are
represented in the branching diagram of Figure 1, which depicts
y2(0)=X'(0) versus 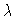 .
One nontrivial bifurcation point is found for
.
One nontrivial bifurcation point is found for
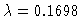 ,
y2(0)=6.275.
,
y2(0)=6.275.
Figure 1
Bifurcation diagram X'(0) versus 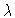
Postscript-Files for better printing results:
This Example
Figure 1
